What is the ExoDx Prostate Test?
The ExoDx Prostate test is a simple, non-invasive urine test to assess your risk of having clinically significant or high-grade prostate cancer. The ExoDx Prostate Test does not require a digital rectal exam (DRE) and provides an individualized risk score that can help determine whether to proceed or defer a prostate biopsy. Use our Physician Finder Map to locate a physician who can order the test for you. The ExoDx Prostate Test is now available for At-Home Collection.

Are You:
- A man aged 50 or older?
- Have a prostate-specific antigen (PSA) level between 2 and 10 ng/mL?
- Speaking with your doctor or concerned about an initial or repeat prostate biopsy?
- Interested in a TeleHealth solution through the At-Home Collection Kit?
- Watch our video here in English, or in Español.
If yes, The ExoDx Prostate Test may be right for you. Learn about our ExoCARES program to see if your insurance plan covers the test.

What are the benefits of the ExoDx Prostate Test (EPI)?
The ExoDx Prostate Test provides a risk assessment for finding clinically significant prostate cancer that otherwise relies on biopsy in men with an elevated PSA. Introducing the ExoDx Prostate (EPI) Test. The EPI Test provides a simple, painless, and accurate answer to inform the prostate biopsy decision. If the test result indicates that a “low risk”, then the man and his doctor (using all other clinical information including family history, prior PSA, abnormal DRE) may decide to defer prostate biopsy – avoiding the pain, complications and costs associated with a prostate biopsy. Interested in learning more? Find a physician or ask about our ExoCARES program by calling 617-588-0500.
Confidence and Peace of Mind
The ExoDx Prostate Test can help inform the physician-patient prostate biopsy decision:
- Non-invasive, simple urine collection, can occur at any time of day
- At-Home Collection Kit can be ordered by your physician and sent directly to your home
- Offers a risk score that informs the prostate biopsy decision
- Clinically proven in men age 50 or over, with PSA levels 2-10ng/mL, and considering an initial or repeat prostate biopsy
- Included in the 2019 National Comprehensive Cancer Center Network Guidelines (NCCN)

**The ExoDx Prostate test is a simple, non-invasive urine test that provides valuable information for the biopsy decision.**
The EPI Test works by analyzing prostate cancer-specific genomic biomarkers that are highly expressed during tumor cell growth found in the urine. These biomarkers are measured in a clinical laboratory and a validated algorithm calculates a score to determine a man’s risk of having aggressive or clinically significant prostate cancer.
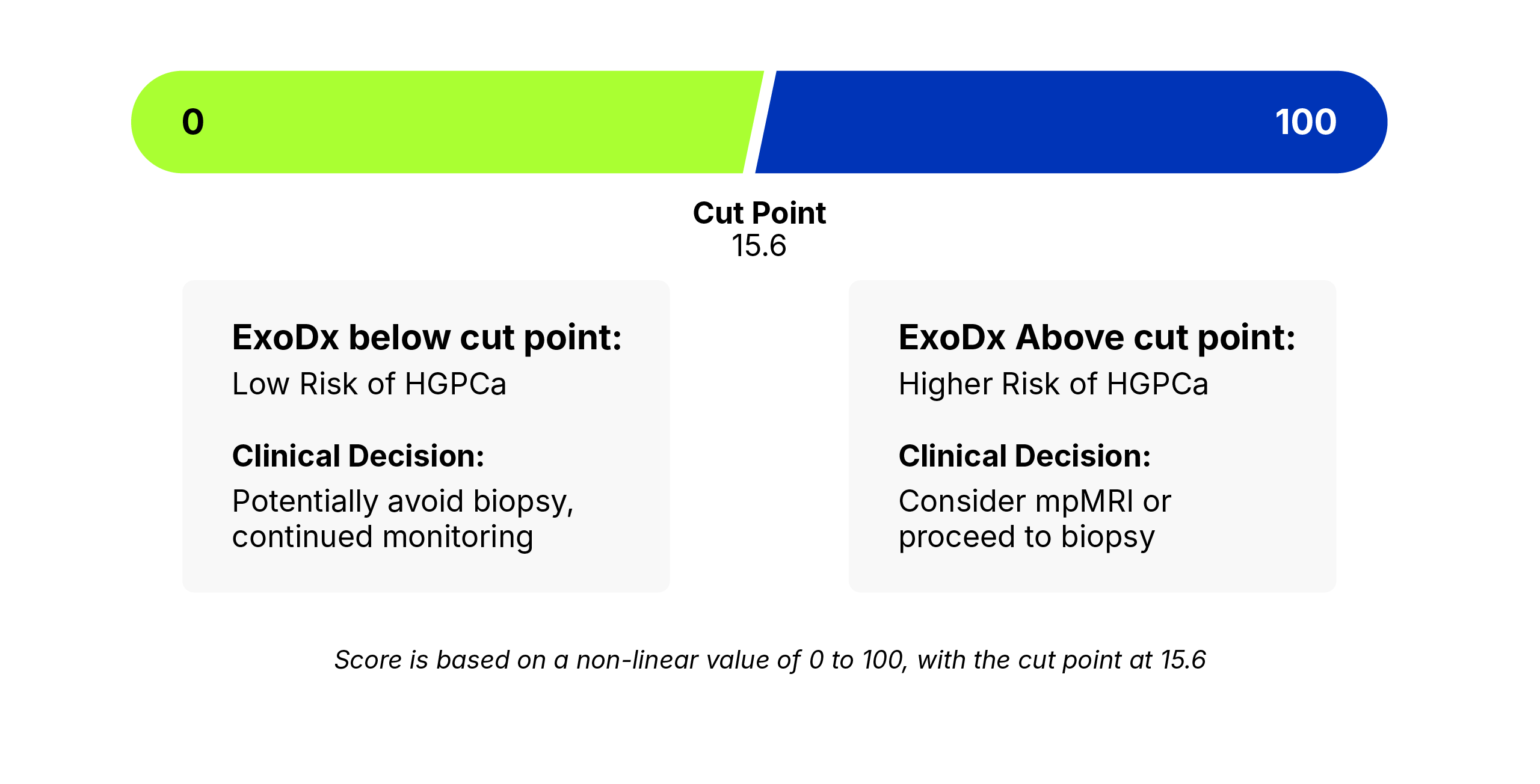
If your ExoDx IntelliScore is: < 15.6 this means lower risk prostate cancer on biopsy, or benign
The intervention is: Potentially avoid biopsy, Continued monitoring
If your ExoDx IntelliScore is: > 15.6 this means higher risk of high-grade prostate cancer on biopsy
The Intervention is: Potentially proceed to biopsy or MRI
Should I Get The ExoDx Prostate Test?
If you are a man over the age of 50 with a prostate specific antigen (PSA) of 2-10 ng/mL and are considering a prostate biopsy, then you should talk to your doctor or health care provider about The ExoDx ProstateTest.
The ExoDx Prostate Test helps physicians and patients make critical decisions about whether or not to proceed to or defer prostate biopsy.
The ExoDx Prostate Test:
- Simple, non-invasive urine test
- Included in the May 2019 NCCN guidelines
- Granted Breakthrough Designation by the FDA
- Covered by Medicare
- Performed in more than 70,000 men
- Proven to avoid almost 30% of biopsies
Common Reasons for a Prostate Biopsy
A prostate biopsy is used to diagnose prostate cancer. Your doctor may recommend a prostate biopsy if any of these prostate cancer symptoms are present:
- Family history of prostate cancer
- Elevated or high PSA
- Increasing PSA over time
- Abnormal results from a digital rectal exam (DRE)
Know before you go: The ExoDx Prostate test is a simple urine test that can inform if prostate biopsy is right for you.
Know Before You Go
There is a special collection cup for The ExoDx Prostate Test. Before you provide a urine sample in the doctor’s office, the professional staff will provide instructions for using the specimen collection device. It is important to correctly follow the instructions to ensure that the test sample is not rejected.
Here is a downloadable one-pager with questions to take with you to your doctor’s office:
- How is prostate cancer detected?
- What is my PSA level and history of PSA levels?
- How accurate is the PSA in assessing my risk for high-grade prostate cancer?
- What is the chance of having a negative or undetermined result for prostate cancer if I have a prostate biopsy?
- What is the chance of having a positive result for prostate cancer if I have a prostate biopsy?
- What is the treatment plan if my prostate biopsy results in a “Low Grade” prostate cancer (Grade Group 1 or below)
- What are alternatives to a prostate biopsy for diagnosis of prostate cancer?
- Is there any risk to obtaining The ExoDx Prostate Test before the biopsy?
- Am I a candidate for the ExoDx Prostate Test?
- Will I be able to use the At-Home Collection Kit?