The ExoDx™ Prostate Test & mpMRI – A Complementary Approach
Click below to visit our detailed webpage and to download a comprehensive, well-referenced Whitepaper on the value of combining biomarker testing and multiparametric magnetic resonance imaging (mpMRI) as complementary methods for more accurate risk assessment and more informed decision-making in the early detection of prostate cancer (PCa).

The management of prostate cancer risk and early detection involves a number of steps, diagnostic procedures and risk assessment methods, typically beginning with a prostate specific antigen (PSA) screening test. Although the PSA test is useful in providing an indication that there may be some risk of PCa, it is well-recognized that the test has many limitations, and that additional follow-up assessment tools are often needed. The limitations of the PSA test include:
- Low sensitivity and specificity for PCa. This means that it may not show an elevated results in all cases where there is risk of PCa, and that in some cases where there is an elevated result there may be no significant risk.
- PSA levels can be increased by a number of other conditions not related to PCa risk
- PSA cannot distinguish between high-grade prostate cancer (HGPCa) and low-grade cancer, and is not prostate cancer specific.
- When the PSA level is in the “gray zone” (2-10 ng/mL) other clinical factors and diagnostic tests or procedures are frequently needed to effectively assess the risk and recommend next steps.
Two of the additional diagnostic tests and procedures that play an important role as follow-up to PSA for more accurate risk assessment and more informed decision-making are mpMRI and biomarkers, such as the ExoDx Prostate Test (also known as EPI).
Introduction to the ExoDx Prostate Test + mpMRI
The clinical question before us is how do we enable the best balance, helping to increase compliance with the biopsy recommendation for patients at higher-risk while providing greater assurance for low-risk patients in an informed shared decision to defer biopsy? There is a vital need to understand better how mpMRI and the various biomarkers can be integrated into the prostate cancer diagnostic algorithm.
Here, we review the potential strengths and limitations of multiparametric magnetic resonance imaging (mpMRI) and how biomarkers, specifically the ExoDx Prostate (EPI) Test might be considered, in combination with imaging, to provide the most informed decision-making.
Potential clinical strategies for combining the ExoDx Prostate Test and multiparametric magnetic resonance imaging (mpMRI). Using the ExoDx Prostate Test either before or after mpMRI are possible approaches for layered risk assessment and more informed decision-making.
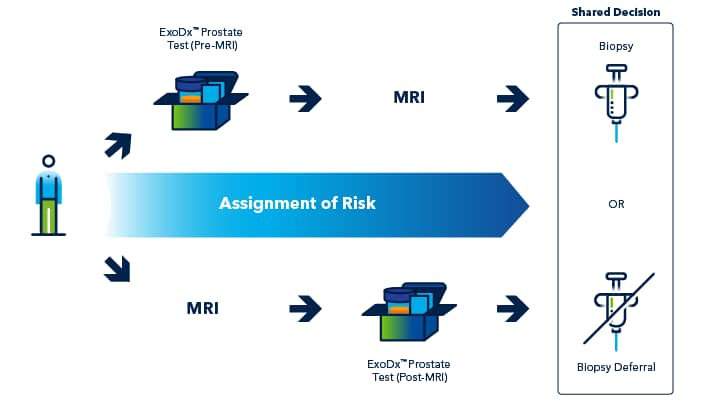
The right patient at the right time for biopsy
Importantly the increased detection of prostate cancer has led to over-treatment of indolent clinically insignificant prostate cancer and performance of large numbers of negative biopsies. The ExoDx Prostate Test utilized as a biomarker risk stratification is emerging as an important aspect of clinical practice and utility in the shared decision-making discussion on biopsy.

