The ExoDx™ Prostate (EPI) Test Methods & Limitations
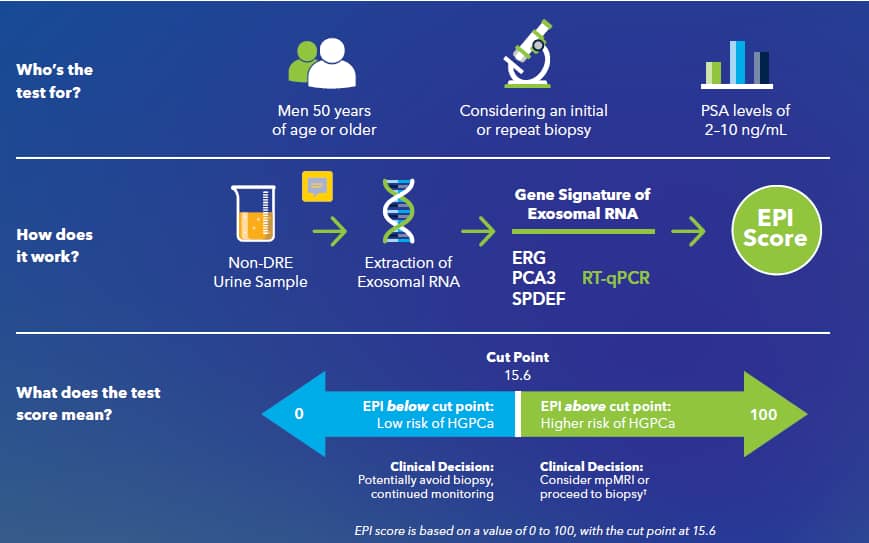
The ExoDx Prostate Test, or EPI (ExoDx Prostate Intelliscore Test) is a non-DRE urine-based liquid biopsy test indicated for men 50 years of age and older with a PSA 2 – 10 ng/mL being considered for an initial prostate biopsy¹ or repeat biopsy due to prior negative biopsy.¹-⁵For this population, the test returns a risk score that predicts the presence of high-grade (Gleason score ≥7) prostate cancer. The cut-off values for other populations, for instance men less than 50 years of age or with previous biopsies, are unknown. The risk score is calculated based on a proprietary algorithm that combines the relative weighted expression of a three-gene signature on exosomal RNA.

The ExoDx Prostate Test At A Glance
The ExoDx Prostate Test Performance
The ExoDx Prostate test had a clinical sensitivity of 92% and a Negative Predictive Value (NPV) of 91% with a cutoff of 15.6.¹ These results cannot be interpreted as absolute evidence of the absence of malignant disease and physicians should utilize this result only in conjunction with other standard of care prognostic information to determine whether to proceed with a tissue biopsy.
.3% Sensitivity⁵
.1% Negative Predictive Value (NPV)⁵
% Negative Predictive Value (NPV) for ruling out ≥ GG3 and higher⁵
.6 Cutoff of the ExoDx Prostate Test¹⁻⁵
Peer-Reviewed Publications to Reference
- 2016 Foundational JAMA Oncology Study Establishing the ExoDx Prostate Test
McKiernan et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy, Journal of American Medical Association (JAMA) Oncology, March 31, 2016. - Prospective Adoptive Utility Study on the ExoDx Prostate Test
McKiernan et al. A Prospective Adaptive Utility Trial to Validate Performance of a Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer in Patients with Prostate-specific Antigen 2–10 ng/ml at Initial Biopsy. European Urology | September 2018. - Clinical Utility of the ExoDx Prostate Test in men presenting for initial Biopsy
Tutrone et al. Clinical utility of the exosome based ExoDx Prostate (IntelliScore) EPI test in men presenting for initial Biopsy with a PSA 2–10 ng/mL. Prostate Cancer and Prostatic Diseases | May 2020.
- Utility of the ExoDx Prostate Test in men with prior negative prostate biopsy and undergoing a repeat biopsy.
McKiernan et al. A urine-based Exosomal gene expression test stratifies risk of high-grade prostate Cancer in men with prior negative prostate biopsy undergoing repeat biopsy, BMC Urology, 2020. - Clinical Performance Evaluation in Three Independent Prospective Studies
Margolis et al. Predicting high-grade prostate cancer at initial biopsy; clinical performance of the ExoDx (EPI) Prostate Intelliscore in three independent prospective studies, Prostate Cancer and Prostatic Diseases, September 30, 2021.
